Quality Assurance
Quality at CliniSafe is built in.
CliniSafe uses an Integrated Management System encompassing GAMP, Quality Management and Information Security Management.
Effective whilst not restrictive, our Integrated Management Systems gives a high degree of control of processes, yet work in a flexible manner for efficient collaboration on global clinical trials.
QA has an independent position at CliniSafe
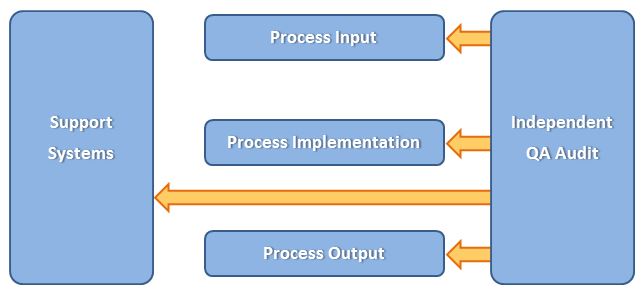
Integrated Management System
The CliniSafe Integrated Management System is blended from GAMP 5, ISO 9001 and ISO 27001 - yet still conforms to the exacting requirements of each.
With an emphasis on 4th generation, process based, management system - procedures can be revised quickly, without the debilitating formalized process entrenched in some larger organisations.
With around 150 Policies, Process diagrams, SOPs and Work Instructions, important aspects of include:
- Document Control
- Change Control - particularly software
- Configuration Control - of hardware
- Testing, Validation and Reporting - of software
- Backup and Restore
- Business Continuity
- Internal and External Auditing
Study Quality Management
Particular emphasis is placed on the quality control of studies, CliniSafe has a carefully built set of Processes and SOPs which cover the running of a study using CliniSafe.
These SOPs will be used when CliniSafe is tasked with any role on a study - and these SOPs are also published "as is" on the Integrated Research Platform - should any other organisation, (e.g. Sponsor or CRO), wish to use them.
Internal Audits
Regular Internal Audits check all business processes
The QA department work actively with operational management in providing oversight of the whole business.
External Audits
The GAMP processes have been externally audited.
BSI Audit the ISO 9001 Quality Management System.
External audits by regulators and clients alike are welcomed.
Training
Staff are regularly trained and coached on critical areas requiring quality compliance. GCP training is also conducted periodically on all staff.
Client Training on the CliniSafe drug checking technology is equally important, with built in eLearning modules for Monitors, Investigators and Integrated Research Platform users. Web based training and on-site training courses are also available.
Customer Feedback
Customer feedback is obtained from Sponsors, CROs, Monitors and Investigators, then trended and discussed with quality targets at Management Review Meetings.
