Welcome to CliniSafe
Awards winning - Drug checking technology for global clinical trials.
We're not the only ones excited about CliniSafe...
Awarded Best Technology Development in Clinical Trials at SCRIP 2010.
E Business of the year, Creative Business of the year and
Enterpeneur of the Year finalist at BIBAs 2012

DIGITISE YOUR DRUG RULES
CliniSafe takes the written drug rules from your protocol and converts them into electronic drug rules. These arethen used to check subjects’ drugs and gives real time feedback, leading to improved patient health, data qualityand reduced costs. CliniSafe is accessible anywhere in the world, across many devices, which means you do notneed to carry the paper protocol with you.

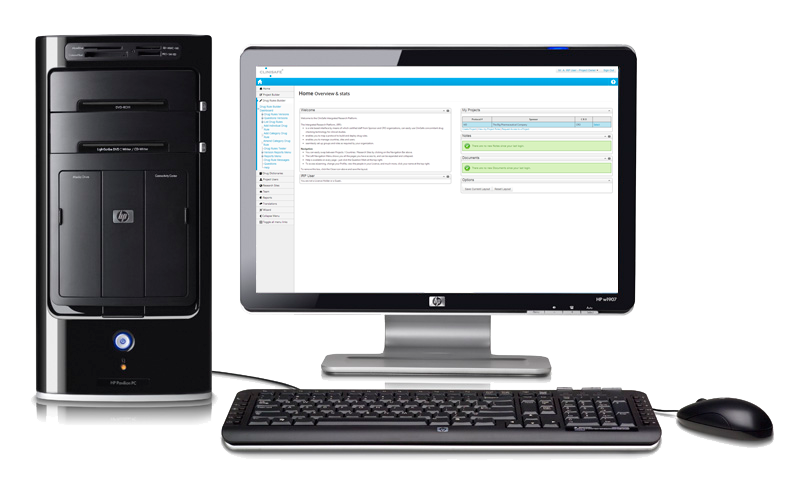
EASY STUDY SETUP
All aspects of setting up and running studies are handled through the Integrated Research Platform. To facilitate collaboration, the CliniSafe Integrated Research Platform provides a “by invitation” security model, which enables company personnel and external specialists to be invited onto a Project, with appropriate security rights.

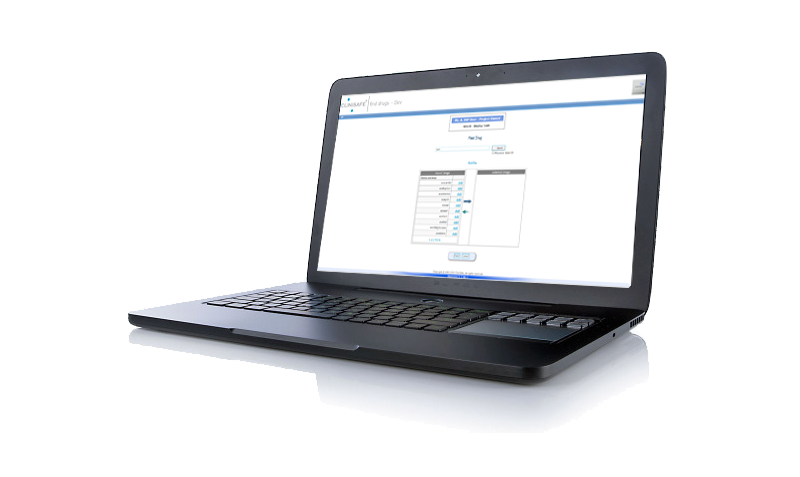
WIZARD
The Drug Checking Wizard is what your Monitors and Investigators see, and everything here is translated. Asimple interface helps the Investigator select all of the drugs that the subject is taking. Once the subject hasbeen added, their medications are recorded to be viewed later, or retrieved again at a later appointment.
The Wizard then displays the Results – which clearly indicates which medications are safe to take for theClinical Trial, and more importantly, which medications are not.

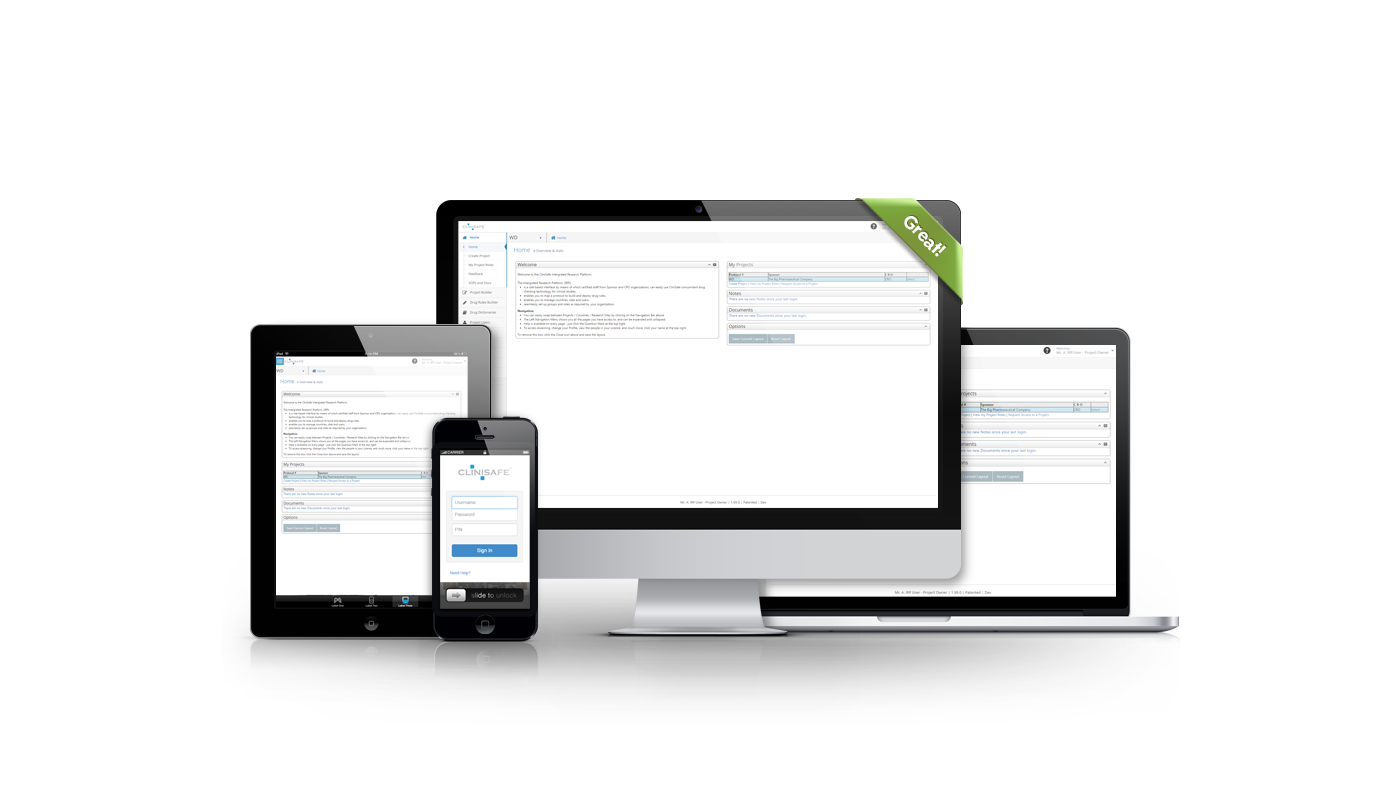
ACCESS FROM ANYWHERE
Our responsive design allows you to check into CliniSafe on your PC, tablet, or mobile phone. So you can still beproductive wherever you are.

Want to find out more about CliniSafe?
We have a full brochure to download or print.
Download the Brochure
New Zealand Patent

New Zealand awards a Patent to CliniSafe for concomitant drug checking over the internet.
CliniSafe exhibits at DIA

CliniSafe Exhibits at the prestigious Drug Information Association (DIA) exhibition in the Hague on the 8th October 2012.
CliniSafe finalists in BIBAs 2012 awards

Customer Service is at the HEART of what we do.
Institute of Directors honours CliniSafe CEO

Chairman and CEO of CliniSafe, Dr Mark Dale, has been named “Most Innovative Director of the Year” at this year’s North West Institute of Directors awards. The award ceremony, held…
CliniSafe shortlisted for Bionow award

Bionow, the North West’s biomedical industry support organisation has shortlisted CliniSafe in the category of “Biomedical Product or Service of the Year”. The Bionow Awards, to be held at the…
Testimonials
"CliniSafe allows me very easily to manage complex protocol drug rules.
"Professor Chris McWilliam
Investigator - UK
Testimonials
"Inadvertent protocol violation in clinical trials due to concomitant use of medicines contraindicated by the protocol can affect around 5% of clinical trial patients per year."
Stephen Goundrey-Smith
Healthcare IT Pharmacist - Royal Pharmaceutical Society of Great Britain
